The healthcare landscape is undergoing significant strain. Escalating costs, aging populations demanding more complex care, the sheer volume of medical data generated daily, and rising levels of clinician burnout are creating systemic pressures. AI has emerged as a potential catalyst for fundamental change, offering pathways to improve efficiency, accuracy, and patient outcomes in this challenging environment.

Within the broad field of AI, one particular approach generates immense excitement and drives tangible advancements: deep learning in healthcare. Far more than just a buzzword, deep learning represents a powerful subset of machine learning (ML) capable of analyzing intricate medical data, from complex images to subtle patterns in patient records. Its ability to learn directly from vast datasets promises to unlock new insights, automate complex tasks, and, ultimately, personalize care at scale. Understanding the capabilities and implications has become increasingly vital for anyone who navigates the future of medicine and health tech.
This article will explore deep learning and how it is explicitly applied within healthcare. We will differentiate it from traditional medical AI methods, examine compelling real-world applications, and discuss the critical data requirements and compliance considerations. Let’s start!
SPsoft’s team can help you overcome challenges and develop innovative deep learning solutions. Let’s discuss how we can leverage our expertise to improve patient outcomes and drive efficiency in your organization!
Deep Learning as The Engine of Modern Healthcare AI
Deep learning is a specialized form of machine learning built upon artificial neural networks with multiple layers, often referred to as “deep” architectures. Inspired loosely by the structure and function of the human brain’s interconnected neurons, which process information in hierarchical layers, these networks learn to identify patterns and relationships directly from raw data.
Instead of being explicitly programmed with rules, DL models learn by example, adjusting internal parameters through exposure to vast amounts of information. A key characteristic is their ability to learn hierarchical representations. Initial layers might detect simple features, while deeper layers combine these features to recognize more complex concepts.
This layered approach makes deep learning exceptionally well-suited for processing the diverse and often unstructured data prevalent in healthcare. Consider the range:
- Medical Images. Pixel values in X-rays, CT scans, MRIs, digital pathology slides, and retinal photographs.
- Genomic Data. Long sequences of DNA or RNA information.
- Physiological Signals. Time-series data from ECGs, EEGs, or continuous glucose monitors.
- Clinical Notes. Unstructured text containing physician observations, patient history, and diagnostic reasoning.
Deep learning models can ingest these data types and automatically learn the most relevant features for a given task (for example, detecting a tumor or predicting disease risk). Therefore, automated feature learning is a cornerstone of deep learning in healthcare, enabling complex data analysis at an unprecedented scale and granularity.
How Deep Learning Differs from Traditional AI in Medicine
While often used interchangeably in popular discourse, deep learning represents a tremendous evolution from traditional AI and machine learning techniques previously standard in medicine. Analyzing these differences is crucial for appreciating the unique capabilities driving current advancements in AI and deep learning in healthcare.
The most fundamental distinction lies in feature engineering. Traditional ML algorithms (like support vector machines or logistic regression) typically require domain experts to manually identify and extract relevant features from the raw data before feeding it to the model.
For instance, to build a model predicting heart attack risk from EHR data, experts must explicitly define features like age, cholesterol levels, blood pressure readings, smoking status, etc. This process is time-consuming, relies heavily on existing knowledge, and may miss subtle, complex patterns hidden within the data. DL, conversely, excels at automated feature extraction. The deep network architecture directly learns the relevant features from the data during training.
That is advantageous when dealing with complex, high-dimensional data like medical images or genomic sequences, where the critical predictive features are not intuitive to human experts. This automated capability accelerates development and may uncover novel biomarkers or diagnostic indicators previously unrecognized.
Another key difference is data handling. Deep learning models generally thrive on large datasets and are particularly adept at processing unstructured data like images and text. Traditional methods often perform better with smaller, structured, tabular datasets. They may struggle to effectively leverage the richness of unstructured information without significant pre-processing and feature engineering. Since a vast amount of clinical information exists in unstructured formats, deep learning’s ability to analyze this data directly reaches significant potential.
Finally, deep learning models have demonstrated superior performance in specific complex tasks, like those involving pattern recognition in images or natural language processing. This advantage is a major driver behind adopting deep learning applications in healthcare for tasks like radiological image analysis and clinical text interpretation. However, this power comes with issues, notably the need for substantial computational resources and large, high-quality datasets. Furthermore, deep learning models’ complex, multi-layered nature can make them less interpretable than traditional methods – the so-called “black box” problem.
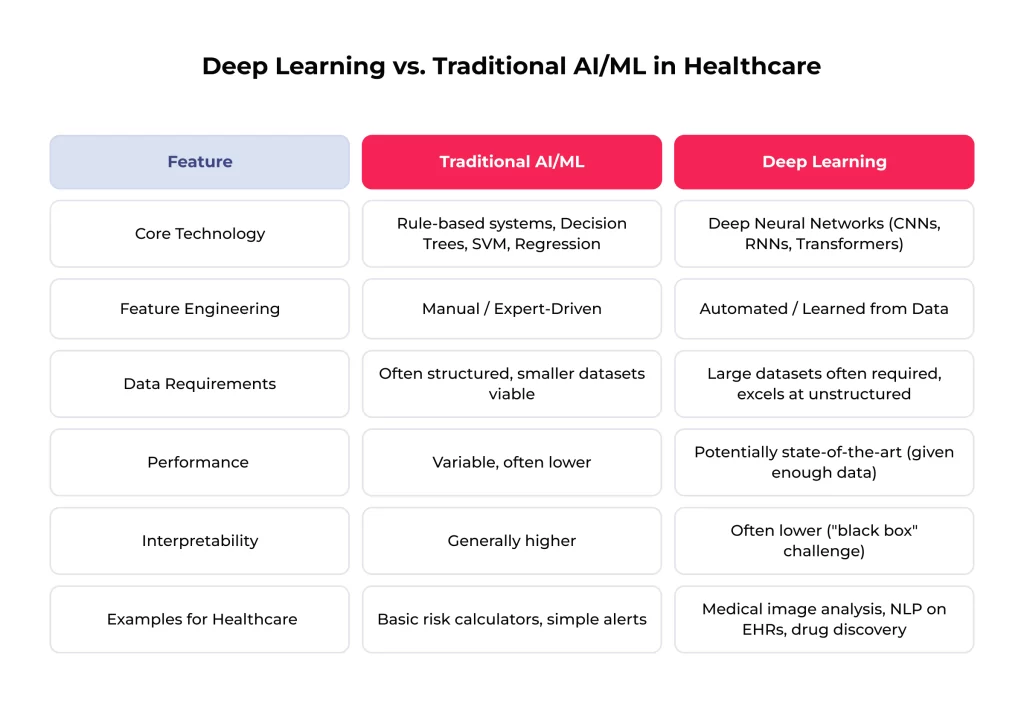
This comparison shows how deep learning in healthcare moves from systems reliant on explicit human knowledge encoding to systems that learn complex patterns directly from data. That enables novel applications and potentially higher performance on challenging medical tasks.
Real-World Deep Learning Applications in Healthcare
The theoretical potential of deep learning is rapidly translating into tangible use cases across healthcare. Such tools are increasingly being developed, validated, and deployed to assist clinicians, researchers, and administrators. Here are some key areas to consider:
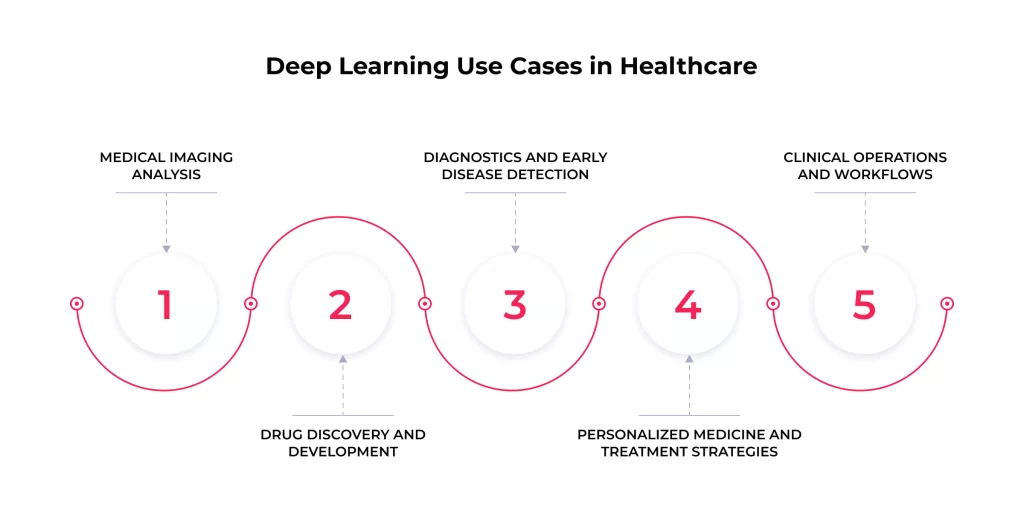
Medical Imaging Analysis
The most mature area for deep learning in healthcare is medical image analysis. DL models, mainly Convolutional Neural Networks (CNNs), excel at identifying complex patterns in visual data. They are applied to various imaging modalities, including X-rays, CT scans, MRIs, ultrasound, digital pathology slides, and retinal scans.
Use case examples:
- Detecting diabetic retinopathy from retinal fundus images
- Identifying potentially cancerous pulmonary nodules on chest CT scans
- Classifying suspicious skin lesions from photographs
- Segmenting tumors and organs-at-risk for radiation therapy planning
- Analyzing digital pathology slides to detect cancer cells or quantify biomarkers
Studies have shown that deep learning models can achieve accuracy comparable to, or even exceeding, that of experienced human radiologists or pathologists in many well-defined tasks. That suggests the potential for improving diagnostic accuracy, reducing inter-observer variability, and significantly speeding up the review process, alleviating workload pressures.
Drug Discovery and Development
Bringing a new drug to market is notoriously slow and expensive. DL offers powerful tools to streamline this process. Models can analyze vast databases of molecular structures, biological pathways, and experimental data. This helps predict how potential drug compounds might interact with targets in the body and their efficacy, toxicity, and pharmacokinetic properties.
Use case examples:
- Identifying promising drug candidates from millions of possibilities
- Designing novel molecules with desired properties in silico
- Predicting clinical trial outcomes
- Stratifying patient populations to identify those likely to respond to a particular therapy
Deep learning can substantially reduce the time and cost associated with pharmaceutical R&D by making drug discovery more targeted and predictive. This may lead to faster availability of novel therapies. While the full impact is longer-term, the activity in this space is intense.
Diagnostics and Early Disease Detection
Beyond imaging, deep learning applications in healthcare extend to broader diagnostics and the crucial goal of early disease detection. Models analyze diverse data streams, such as structured and unstructured data within EHRs, lab results, genomic sequences, wearable sensor data, and even subtle changes in voice patterns or facial expressions.
Use case examples:
- Predicting the onset of sepsis in hospitalized patients based on patterns in vital signs and lab results from EHR data
- Identifying individuals at high risk of developing Alzheimer’s disease years before clinical symptoms manifest by analyzing brain scans or cognitive assessments
- Detecting arrhythmias like atrial fibrillation from ECG signals captured by smartwatches and flagging potential adverse drug events based on clinical notes
Early detection is often crucial for effective treatment. Deep learning can improve patient prognoses and quality of life by identifying diseases like cancer or neurodegenerative conditions at earlier, more treatable stages.
Personalized Medicine and Treatment Strategies
The “one-size-fits-all” approach to medicine is increasingly giving way to personalized strategies tailored to individual patients. Deep learning is a key enabler of this shift. Models integrate vast amounts of heterogeneous information for a single patient, including unique genomic profiles, clinical history, lifestyle factors, imaging data, and real-time monitoring data.
Use case examples:
- Predicting which cancer patients will respond best to specific chemotherapies or immunotherapies based on their tumor’s genetic makeup and other biomarkers
- Optimizing drug dosages to maximize efficacy while minimizing side effects
- Identifying patients who would derive the most benefit from prophylactic interventions
- Developing highly individualized treatment plans.
That leads to more effective treatments, reduced adverse effects, and better overall patient outcomes by moving from population averages to truly individualized care. The synthesis of diverse data types is where DL truly shines, creating a comprehensive patient understanding beyond human capacity. However, it also underscores the significant data integration challenges that must be overcome.
Clinical Operations and Workflows
Deep learning in healthcare is not limited to direct clinical applications; it also offers tremendous potential for improving the efficiency and effectiveness of healthcare operations.
Use case examples:
- Predicting patient admissions and emergency department wait times to enhance resource allocation
- Optimizing hospital bed management and patient flow
- Automating aspects of medical billing and coding by analyzing clinical documentation
- Improving surgical scheduling
- Predicting hospital-acquired infections to enable targeted prevention efforts
These applications can lead to substantial cost savings, reduced administrative burden on clinical staff, better patient experience and overall utilization of scarce healthcare resources.
However, it is essential to note that these applications’ maturity and regulatory readiness vary. Imaging analysis tools often have clearer validation pathways and established regulatory precedents. Meanwhile, applications involving complex predictions like personalized treatment response or operational optimization may face longer timelines for widespread adoption.
The Data Foundation: Fueling Deep Learning Models
DL models are powerful, but they are fundamentally data-driven. The quality, quantity, and nature of the data used to train and validate them are paramount to their success and safety in the sensitive healthcare domain. Understanding the data requirements and challenges is essential for anyone developing or implementing deep learning in healthcare.
Essential Data Types for Healthcare Deep Learning
Deep learning models in healthcare leverage a wide variety of data sources, often combining multiple types to build complex models:
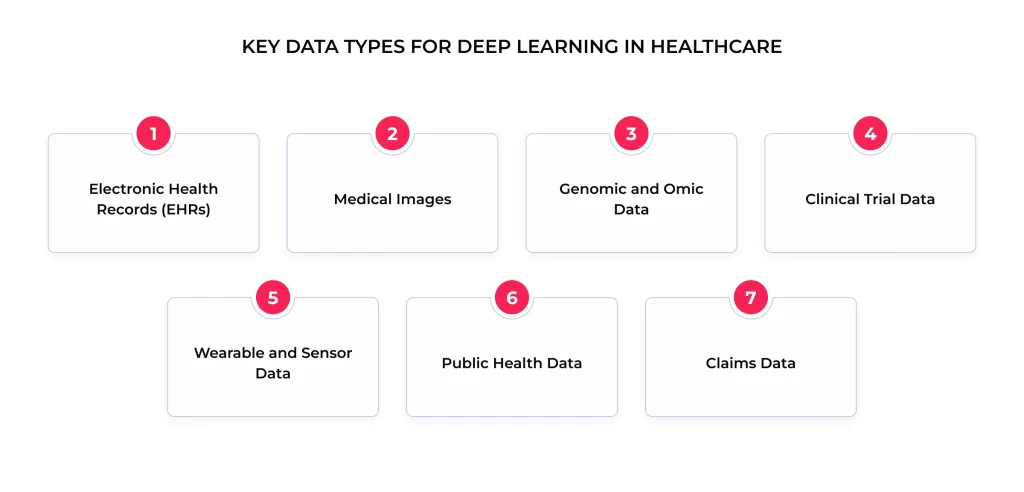
- Electronic Health Records (EHRs). Rich sources containing patient demographics, diagnoses, procedures, medications, lab results, vital signs, and clinical notes.
- Medical Images. Data in standard formats like DICOM from modalities like X-ray, CT, MRI, PET, ultrasound, and digital pathology.
- Genomic and Omic Data, including DNA/RNA sequences, gene expression data, proteomics, and metabolomics, provide insights into individual biological makeup and disease mechanisms.
- Clinical Trial Data: Structured data from research studies are crucial for developing models related to drug efficacy and treatment response.
- Wearable and Sensor Data. Continuous physiological data streams (heart rate, activity levels, sleep patterns, glucose levels) from consumer wearables and medical-grade sensors.
- Public Health Data. Aggregated data on disease prevalence, environmental factors, and population health trends.
- Claims Data. Information on healthcare utilization and costs is useful for operational and health economics modeling.
DL’s ability to process and find patterns within these diverse and often unstructured data types is a key advantage, but it also necessitates careful data management and preparation.
The Critical Role of Data Quality, Quantity, and Diversity
Simply having data is not enough; three factors are critical for effective deep learning in healthcare:
- Quantity. Deep learning models, with their millions or even billions of parameters, are notoriously data-hungry. They require large datasets to learn complex patterns effectively and generalize to new, unseen data. Insufficient data can lead to models that are “overfitted” to the training set and perform poorly in real-world scenarios.
- Quality. The “garbage in, garbage out” principle applies strongly. Models trained on inaccurate, incomplete, or poorly labeled data will inevitably produce unreliable results. Standard quality issues in healthcare data include missing values, inconsistent terminology across different systems, measurement errors, and inaccurate labeling. Ensuring data quality requires rigorous cleaning, validation, and curation processes.
- Diversity is the most critical aspect from an ethical and clinical standpoint. Training data must adequately represent the diversity of the patient population on which the model will be used. This encompasses variations in age, sex, gender identity, ethnicity, geographic location, socioeconomic status, and comorbidities. Models trained on narrow or biased datasets may perform poorly or unfairly for underrepresented groups, potentially perpetuating or exacerbating health disparities.
Overcoming Data Challenges: Silos, Standardization, and Privacy
Despite the vast amount of healthcare data generated, accessing and utilizing it for deep learning faces significant hurdles:
- Data Silos. Healthcare information is often fragmented and stored in disparate systems within different departments or across different institutions (hospitals, clinics, labs). The latter may be unable or unwilling to share data due to technical incompatibilities, competitive concerns, or regulatory interpretations. That makes assembling the large, integrated datasets needed for training powerful models incredibly difficult.
- Lack of Standardization. Data is often recorded using various formats, terminologies, and coding systems (e.g., different versions of ICD codes and proprietary EHR field names). This lack of standardization hinders data aggregation and requires effort in mapping and harmonization before datasets from different sources can be effectively combined.
- Privacy Concerns. Healthcare data contains highly sensitive Protected Health Information (PHI). Strict regulations like HIPAA in the US (and similar regulations globally) govern its use and disclosure. While de-identification techniques are used, ensuring robust anonymization while retaining data utility for complex deep-learning models is challenging. That is because models become more adept at finding patterns that could potentially re-identify individuals from seemingly anonymized data.
Overcoming these challenges necessitates industry-wide collaboration on data standards and clear regulatory guidance that balances innovation with patient privacy. Progress hinges on building trust and establishing governance structures that facilitate responsible data access for advancing deep learning in healthcare.
Future Outlook: What Is Next for AI and Deep Learning in Healthcare?
The field continues to evolve rapidly. Key trends shaping the future include:

- Multimodal Learning. Developing models that can integrate and learn from multiple data types simultaneously (e.g., combining imaging, genomics, and clinical notes) for a more holistic patient understanding.
- Federated and Privacy-Preserving AI. Increased use of techniques like federated learning to train models on distributed datasets without compromising patient privacy, overcoming data access barriers.
- Edge AI. Deploying AI models directly onto medical devices or local hardware for real-time analysis and decision-making without needing constant cloud connectivity.
- Reinforcement Learning. Exploring the use of reinforcement learning for optimizing complex, sequential decisions, such as dynamic treatment adjustments based on patient response.
- AI Regulation. Expect continued development and refinement of regulatory frameworks (like those from the FDA and international bodies) specifically addressing AI’s validation, monitoring, and ethical use in healthcare.
- Market Growth. Industry forecasts consistently predict substantial growth in the market for AI and DL in healthcare, indicating strong ongoing investment and perceived value.
The future success of AI and deep learning in healthcare will likely depend less on isolated algorithmic breakthroughs and more on building collaborative ecosystems. That involves fostering data-sharing initiatives, developing common standards, and promoting interdisciplinary collaboration between technologists, clinicians, ethicists, and regulators.
Final Thoughts
Deep learning in healthcare stands as one of our time’s most transformative tech forces, offering great potential to reshape medical research, clinical practice, and healthcare operations. From enhancing diagnostic precision in medical imaging to accelerating drug discovery, its capabilities promise to address some of modern medicine’s most pressing challenges.
However, realizing this immense potential is not automatic. It requires a clear-eyed view of the significant hurdles that remain. Navigating the complexities of data access, quality, and privacy, ensuring seamless integration into clinical workflows, and addressing ethical considerations around bias and transparency are critical prerequisites for success. The path forward demands careful planning, responsible development practices, robust validation, and ongoing vigilance.
Lastly, integrating AI and deep learning in healthcare hinges on collaboration. Clinicians, researchers, data scientists, engineers, ethicists, policymakers, and patients must work together to guide the development and deployment of these powerful tools. By maintaining a steadfast focus on patient-centric applications, we can harness the power of deep learning to build a more intelligent, efficient, and personalized healthcare future.
From developing AI-powered diagnostic tools to creating personalized medicine platforms, we can tailor solutions to your needs. Learn how we can integrate deep learning seamlessly into your healthcare workflows and systems!
FAQ
Can deep learning detect diseases like cancer or Alzheimer’s earlier?
Yes, early disease detection is one of the most promising areas for deep learning applications in healthcare. Deep learning models excel at identifying subtle patterns in complex data that may precede overt clinical symptoms or be easily missed by human observers.
Models can analyze medical images (e.g., mammograms for breast cancer, retinal scans for diabetic retinopathy, brain MRIs for early signs of Alzheimer’s), genomic data for predisposition markers, patterns in EHR data, or even vocal biomarkers.
Deep learning enables timely interventions by flagging potential issues earlier than traditional methods, significantly improving treatment effectiveness and patient outcomes. For example, research has shown promising results in identifying specific cancer types on scans or predicting cognitive decline based on subtle neurological changes. These tools are typically designed to assist clinicians by highlighting areas of concern or stratifying risk, not to replace their judgment entirely. Clinical validation and integration into workflows are key.
How accurate are deep learning models for medical imaging?
The accuracy of deep learning models in medical imaging varies considerably depending on several factors:
– The specific clinical task
– The quality and quantity of the training data
– The specific algorithm used
– How performance is measured
Numerous studies have demonstrated that deep learning models can achieve accuracy levels comparable to human experts for specific, well-defined tasks. High performance is often reported in tasks like classifying diabetic retinopathy, detecting certain fractures on X-rays, or identifying specific types of cancer on pathology slides.
It is crucial to look beyond headline accuracy figures. Validation of diverse, independent datasets that reflect real-world patient populations is essential to ensure generalizability. Performance in controlled research settings may not always translate directly to messy clinical reality. Ongoing monitoring of model performance after deployment is also critical.
Can deep learning integrate with EHR or hospital systems?
Technical integration is possible, but it presents practical and technical challenges. Effective integration is crucial for deep learning in healthcare tools to be useful in clinical practice.
Deep learning outputs could be integrated in various ways:
– Displaying analysis results directly within a radiologist’s Picture Archiving and Communication System (PACS) workstation
– Triggering alerts or notifications within the main Electronic Health Record (EHR) system
– Providing clinical decision support prompts based on patient data
– Automating tasks within administrative systems
Meanwhile, key hurdles include:
– Achieving interoperability between the AI system and existing hospital IT infrastructure (often requiring adherence to standards like HL7 FHIR)
– Mapping data correctly between systems
– Designing integrations that fit seamlessly into established clinical workflows without causing disruption
– Ensuring robust security and HIPAA compliance across integrated systems
– Managing the deployment and updates of AI models within the hospital environment
How do hospitals implement deep learning technologies?
Implementing AI and deep learning in healthcare is a complex, multi-stage process beyond simply purchasing software. A strategic approach is required:
1. Identify Clear Use Case. Start by defining a specific, high-value clinical or operational problem that deep learning can realistically address.
2. Data Assessment & Preparation. Evaluate the availability, quality, and representativeness of the necessary data. That often involves significant effort in data cleaning, standardization, and ensuring compliance frameworks are in place.
3. Model Selection/Development & Validation. Decide whether to purchase a validated solution from a vendor (requiring due diligence) or develop a model in-house (requiring significant expertise). Rigorously validate the chosen model’s performance, safety, and fairness on local data.
4. Technical Integration Planning. Design how the tool will integrate with current systems like the EHR or PACS, considering workflow implications and technical requirements.
5. Pilot Testing & Clinical Validation. Conduct a pilot study in a controlled clinical setting to assess real-world performance, usability, impact on workflow, and clinician acceptance.
6. Training & Change Management. Provide thorough training for clinicians and staff on using the tool effectively and interpreting its outputs. Address concerns and manage the necessary changes to clinical workflows.
7. Regulatory & Compliance. Ensure all necessary regulatory approvals are obtained (e.g., FDA clearance/approval for diagnostic tools) and that the implementation adheres to HIPAA and other relevant regulations.
8. Ongoing Monitoring & Maintenance. Continuously monitor the model’s performance in production, manage software updates and model retraining, track clinical and operational impact, and ensure ongoing value and safety.
Can deep learning make biased or unsafe decisions in healthcare?
Yes, this is a critical risk that must be actively managed. Bias and safety issues can arise from multiple sources:
– Biased Training Data. If the data used to train a model underrepresents certain demographic groups, the model may perform less accurately or make systematically poorer predictions. That can lead to significant health disparities.
– Algorithmic Bias. Choices made during model design and optimization can inadvertently introduce biases.
– Feedback Loops. If AI predictions influence future clinical decisions or data recording, it can create feedback loops that amplify existing biases over time.
– Safety Risks. Beyond bias, unsafe decisions can occur due to model errors, automation bias, unexpected model behavior when encountering data different from its training set, or security vulnerabilities.
Addressing these risks requires rigorous testing for bias and fairness across various subgroups, striving for transparency in model decisions, and adopting robust validation processes. Strong human oversight and clinical judgment must also remain central, and continuous monitoring for performance degradation or unexpected behavior must be conducted.
Who is responsible if a deep learning system makes a wrong diagnosis?
That is a complex legal and ethical question with currently evolving answers. Liability in an AI-driven error is likely to be distributed and context-dependent.
Responsibility could potentially fall upon:
– The clinician who used the AI tool and made the final clinical decision (often, clinicians retain ultimate responsibility);
– The developers of the AI algorithm;
– The hospital or healthcare institution that implemented the system and established protocols for its use;
– Possibly even the providers of the data used to train the AI, if data quality issues contributed to the error.
There is no established legal precedent specifically for harm caused by medical AI. Regulatory frameworks are still developing to address issues like whether AI should be treated as a medical device, a service, or a form of decision support. Clear lines of accountability are still being debated and defined. This uncertainty represents a significant challenge for adoption, particularly for high-stakes applications.
How does deep learning improve patient outcomes?
The ultimate goal of deep learning in healthcare is to improve patient outcomes. That can occur through several pathways:
– Earlier & More Accurate Diagnosis. Detecting diseases like cancer or diabetic retinopathy at more treatable stages.
– Personalized Treatment. Tailoring therapies based on individual patient characteristics (genomics, biomarkers) to increase efficacy and reduce side effects.
– Risk Prediction & Prevention. Identifying high-risk patients who could benefit from preventative measures or closer monitoring.
– Enhanced Surgical Support. Improving surgical planning, intraoperative guidance, or robotic control.
– Reduced Medical Errors. Automating tasks or providing decision support to minimize human error.
– Evidence Base. While many studies show potential, large-scale evidence definitively proving improved patient outcomes across diverse applications and settings is still emerging for many DL applications in healthcare. Demonstrating real-world clinical utility and outcome improvement remains a key focus of ongoing research and validation efforts.
What are the cost-saving benefits of using deep learning in hospitals?
Adopting AI and deep learning in healthcare can lead to cost savings for hospitals and health systems, although realizing these benefits requires careful planning and investment. Potential cost-saving areas involve:
– Improved Operational Efficiency. Faster diagnostic processes, automation of administrative tasks (like coding and billing), reduced manual labor costs.
– Reduced Medical Errors. Lowering costs associated with treating complications arising from diagnostic or treatment errors.
– Optimized Resource Allocation. More efficient staff and operating room scheduling, better management of hospital beds and patient flow, and a waste reduction.
– Enhanced Preventative Care. Early disease detection can reduce the need for costly late-stage treatments and hospitalizations.
– Accelerated Drug Development. More efficient R&D could contribute to lower drug costs in the long term.
Achieving these savings requires substantial upfront investment in tech, data infrastructure, integration, training, and ongoing maintenance. Demonstrating a clear return on investment (ROI) can be challenging, particularly in the short term.